Mathematical Modeling and the Monkey King: Use of modeling for more efficient NHP study design
By: Fei Hua, PhD,
Marc Presler, PhD
Background
Monkeys and humans share a strong bond, reflected not only in a shared genetic heritage, but in history and folklore. In Chinese literature from the 16th century, Journey to the West (Xi You Ji) tells a famous story of a Buddhist monk named Tang Seng during his journey to obtain sacred Buddhist texts. Tang Seng is protected through many trials by the Monkey King, a cunning and powerful companion during the long search for enlightenment and knowledge.
In the modern world, non-human primates (NHP) continue to support humans in our difficult search to improve health with safe and effective therapeutics. Animal models such as the Cynomolgus monkey (Macaca fascicularis), or “cyno” for short, serve as critical preclinical species for the required testing of novel drugs before running clinical studies in patients.
As outlined by the 3Rs principle (Russell and Burch, 1959), it is ethical to reduce the numbers of animals and to refine the experiments used to test the safety and efficacy of drugs. Additionally, the cost of monkey studies has increased dramatically over the past year (Tian, 2021). The soaring price-per-animal is particularly impactful for the smaller, innovative biotechs that strive for capital efficiency in early drug development programs, especially in the tighter fundraising markets.
How Can Mathematical Modeling Assist in Designing Better Experiments?
Here, we share some thoughts from our experience on monkey studies, and how mathematical modeling can assist in designing more informative and efficient experiments.
The first step is to determine if non-human primate is the right tox species for the program. Some considerations include cross-reactivity of the molecule, conservation of the target/pathway biology, immunogenicity etc. In addition to tox evaluation, non-human primates are commonly used species for predicting human pharmacokinetics (PK) of biotherapeutics. Initial studies are typically conducted to explore a range of doses for estimating pharmacokinetic and safety effects. Information collected from exploratory studies inform conditions used in definitive GLP (Good Laboratory Practice) toxicology studies, performed to a quality standard required for regulatory submission. The totality of information gathered from preclinical studies will form the basis to recommend a starting dose and safe use conditions for first-in-human trials.
Well-planned exploratory PK and toxicology studies are critical for designing successful definitive GLP studies. While exploratory experiments should be “lean”, sponsors often try to “do too much” within one experiment, which can later require repeated studies. Borrowing the concept from the clinical trials, identifying primary and secondary goals then organizing the protocol around them will be important when designing exploratory cyno studies.
We have found modeling as a helpful tool to ensure successful achievement of study goals. If the primary objective is to understand toxicity, then the selection of dose, route and dosing interval is important and should mimic the eventual clinical plan as closely as possible. An early prediction of clinical efficacious dose and dosing regimen with mechanistic modeling (Marcantonio et al, 2022) can influence clinical strategy if it is not yet crystalized. For example, having an idea of the eventual efficacious dose can ensure a high enough dose is tested to avoid limiting the later clinical dosing scheme.
If the primary objective is to understand PK and PD, it may motivate much lower doses than would be chosen for a toxicity study. For example, if target-mediated drug disposition (TMDD) is expected for an antibody therapy, a lower dose where the TMDD is not yet saturated should be included. In addition, the team should also consider the appropriate time points to best characterize the molecule’s PK profile. A mechanistic PKPD model that provides a preliminary prediction of the monkey PK profile can be a powerful tool to guide both dose selection and time points selection (Figure 1).
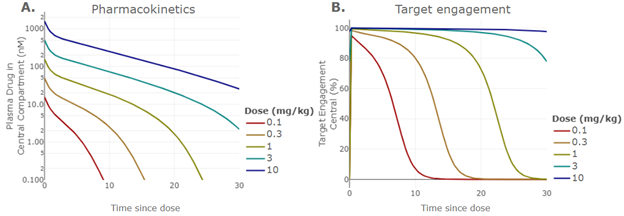
As shown in a simulation in Figure 1A, if selecting doses of 1, 3 and 10 mg/kg, a parallel PK profile is expected over the first two weeks, which contains limited additional information per dose. Instead, administering doses of 0.1, 1 and 10 mg/kg will allow characterization of both TMDD and linear PK. This simulation also informs sampling time selection. For the 1 mg/kg dose shown in Figure 1A, stopping PK collection at week 2 would miss the opportunity to identify non-linear PK. But if PK is collected at week 4, exposure may be undetectable, creating uncertainty at the end of the time series. Adding a PK collection at week 3 will provide substantially more information.
The simulations can also predict the extent of target engagement. In Figure 1B, the simulation predicts full target engagement through at 10 mg/kg dose and minimal differentiation at the 1 and 3 mg/mg doses over the first two weeks post dose. If obtaining dose response of target engagement is one of the primary goals, a lower dose at 0.1 mg/kg should be selected. Alternatively, a later time point of week 4 at 1 mg/kg should be collected along with the 10 mg/kg dose.
How Does This Affect Project Optimus?
Finally, the FDA’s Project Optimus is recommending better understanding of dose response in oncology studies. These efforts require an integrative preclinical program. A monkey study showing dose response in PD and toxicity can be quite helpful data complementing any human data to be collected later on. Modeling efforts can help support these important and costly experiments and better leverage the supporting data package.
Reflecting on Tang Seng Journey to the West, the modern Monkey Kings remain to protect human patients by helping us better characterize the safety and efficacy profiles. Just as the mythical characters on their journey, our own paths in drug development require persistence, teamwork, and the wisdom to align fragmented information into a connected whole.
While preclinical-stage data often exist as isolated pieces, in silico modeling has the power to integrate these data and maximize the knowledge we can extract from these data, from monkey or otherwise, into a unified path forward to improve patient lives.
Sources
Marcantonio, Diana H., Andrew Matteson, Marc Presler, John M. Burke, David R. Hagen, Fei Hua, and Joshua F. Apgar. 2022. “Early Feasibility Assessment: A Method for Accurately Predicting Biotherapeutic Dosing to Inform Early Drug Discovery Decisions.” Frontiers in Pharmacology 13. https://doi.org/10.3389/fphar.2022.864768.
Russell, W.M.S. and Burch, R.L. (1959) The Principles of Humane Experimental Technique.
Tian CY. 2021. “China is facing serious experimental monkey shortage during the COVID-19 lockdown.” J Med Primatol. 50(4):225-227. doi:10.1111/jmp.12528

