Background
- Predicting clinical ADC efficacy and toxicity is a challenge. The mAb, linker, and payload all need to be optimized for a particular tumor target, indication, and patient population.
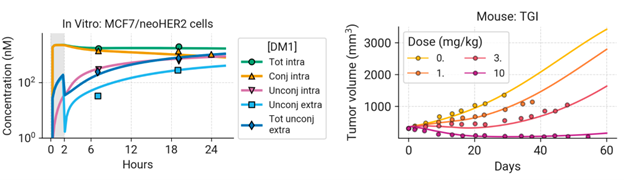
- Two mechanistic models were developed using preclinical Trastuzumab-emtansine (T-DM1) data to project clinical efficacy and tumor reduction and thrombocytopenia (TCP) incidence, a common ADC adverse event.
- The models were built to be generalizable for the study of any ADC.
- Combining efficacy and toxicity models allows us to explore common therapeutic metrics, such as therapeutic window and indexes.
Conclusions
- Clinical outcomes of ADCs can be projected with this mechanistic platform model.
- The efficacy model was validated with T-DXd in mBC patients, which successfully reproduced clinical PK, efficacy, and PSF.
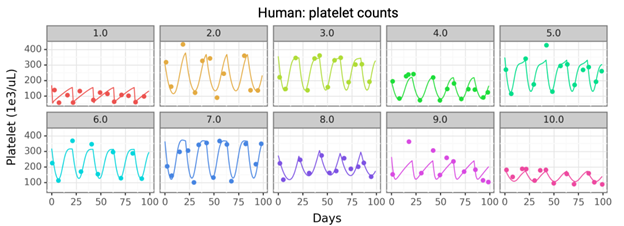
- Mechanistic TCP model can capture interpatient variability.
- The models were built to be generalizable to other ADCs by substituting relevant parameters.
Future directions:
- Continue validating efficacy model on other ADCs with different indications.
- Use TCP rates from clinical data with higher doses to improve predictions. Determine whether the mechanistic TCP model can be used for preclinical to clinical translation.