Introduction
CD3 T-cell engagers, or TCEs, are an active class of drugs in preclinical and clinical development. In 2021, there were 58 CD3 bispecific T-cell engagers in clinical development. While Blinatumomab, a CD19/CD3 bispecific TCE, was approved in 2014 for the treatment of B-cell precursor acute lymphoblastic leukemia, successful development of a CD3 bispecific TCE for solid tumors has lagged behind. Several challenges are associated with targeting solid tumors, including expression of targets on healthy tissues and the potential for on-target, off-tumor toxicity, low numbers of infiltrating T cells, and limited drug penetration into tissues.
Background
Applied BioMath Assess™ can be used to evaluate the risk of on-target, off-tumor toxicities for CD3 bispecific T-cell engagers and to make an early prediction about therapeutic index and projections of effective and tolerable doses for T-cell engagers in solid tumors. In this case study, we will demonstrate the ability to predict the on-target, off-tumor toxicity observed for solitomab. We will use this model to identify properties of tumor-associated antigens that could be more successfully targeted with a TCE. Furthermore, we will explore the feasibility of next generation T-cell engager molecules to enhance tissue selectivity leading to improved therapeutic index (TI). We will explore this in the context of solitomab, an EpCAM/CD3 BiTE and focus on the following questions:
- What is the dose at which we would predict on-target off-tumor toxicity for solitomab?
- What difference in target expression between the tumor and the GI would be necessary for a TCE to have a reasonable therapeutic index?
- Can we achieve tissue selectivity with a next generation T-cell engager design?
Bispecific CD3 T-Cell Engagers Can Redirect T-Cells to Kill Tumor Cells in Solid Tumors
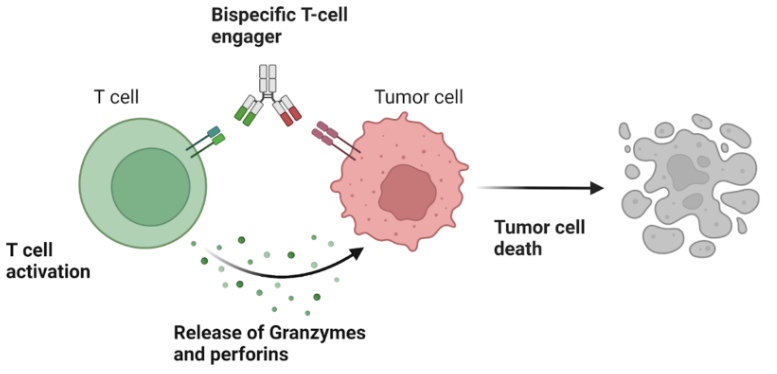
Challenges:
- Normal tissue expression of tumor antigen
- Potential for "on-target off-tumor" related toxicities
- Low numbers of infiltrating T cells
- Limited drug penetration